Our research is focused on the physiology and pathophysiology of skeletal muscle function. It aims at understanding how mechanisms involved in the control of skeletal muscle Ca2++ homeostasis and excitation-contraction (EC) coupling operate under normal and disease conditions. For this we use a combination of molecular biology, biochemistry, in vivo gene transfer and simultaneous electrophysiology and fluorescence detection on cultured cells and on single isolated differentiated muscle fibers.
Since September 2025 our team merged with the group of Binnaz Yalcin whose expertise is focused on the genetics of neurodevelopmental disorders
(https://blog.u-bourgogne.fr/yalcingroup/) and our research plans now include understanding the muscle deficiencies that are associated with these disorders.

TEAM
- Vincent JACQUEMOND
SENIOR RESEARCHER, CNRS - Binnaz YALCIN
SENIOR RESEARCHER, Inserm - Bruno ALLARD
PROFESSOR, UCBL - Christine BERTHIER
ASSISTANT PROFESSOR, UCBL - Stephan COLLINS
ASSISTANT PROFESSOR, UBE - Léa DEMESMAY-MICHAUD
POST-DOC - Jonathan SCHREIBER
ENGINEER - Céline MALLEVAL
ENGINEER - Camille DAUVOIS
Assistant Engineer - Emilia SKUTUNOVA
PhD Student - Xiang WU
PhD Student - Hanzala DAUD
Master Graduate
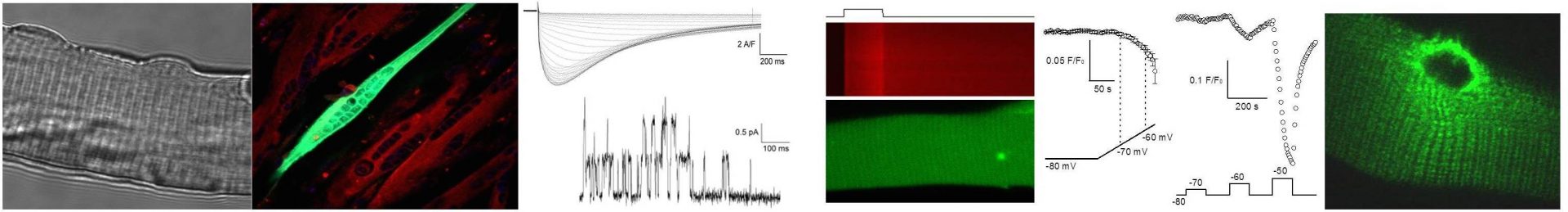 PROJECTS
PROJECTS
Our research is focused on the physiology and pathophysiology of skeletal muscle function. It aims at understanding how specific mechanisms involved in the control of skeletal muscle Ca2+homeostasis and excitation-contraction (EC) coupling operate under normal and disease conditions. Muscle contraction is initiated when action potentials fired at the end-plate of the muscle cells propagate throughout the plasma membrane and trigger a conformational change of the CaV1.1 protein which gates open a Ca2+release channel (type 1 ryanodine receptor, RyR1) in the sarcoplasmic reticulum (SR) membrane. Ca2+then gets released from the SR into the cytosol and triggers contraction. Besides Ca2+release from the SR there is also Ca2+entry from the extracellular medium.
Our main current projects aim at:
1- Understanding basic mechanisms involved in the regulation of CaV1.1 and of RyR1 function.
2- Demonstrating how excitability and/or EC coupling are altered by specific disease mutations affecting the genes encoding CaV1.1, RyR1 and also other proteins involved in the function and/or maintenance of the EC coupling machinery.
The overall project stands on a set of methods and expertise that includes molecular biology and biochemistry, in vivo gene transfer and a state of the art combination of electrophysiology and fluorescence detection on single isolated differentiated muscle cells from mouse.
SELECTED PUBLICATIONS
- Superfast excitation–contraction coupling in adult zebrafish skeletal muscle fibers. Idoux R, Bretaud S, Berthier C, Ruggiero F, Jacquemond V, Allard B. J Gen Physiol (2022) 154 (9): e202213158.
- Lack of the myotendinous junction marker col22a1 results in posture and locomotion disabilities in zebrafish. Malbouyresa M, Guirauda A, Lefrançois C, Salamito M, Nauroy P, Bernard L, Sohm F, Allard B, Ruggiero F. Matrix Biology (2022) 109:1-18
- Mice with muscle-specific deletion of Bin1 recapitulate centronuclear myopathy and acute downregulation of dynamin 2 improves their phenotypes. Silva-Rojas R, Nattarayan V, Jaque-Fernandez F, Gomez-Oca R, Menuet A, Reiss D, Goret M, Messaddeq N, M Lionello V, Kretz C, S Cowling B, Jacquemond V, Laporte J. Molecular Therapy (2022) 30(2):868-880
- Pannexin-1 and CaV1.1 show reciprocal interaction during excitation-contraction and excitation-transcription coupling in skeletal muscle. Jaque-Fernández F, Jorquera G, Troc-Gajardo J, Pietri-Rouxel F, Gentil C, Buvinic S, Allard B, Jaimovich E, Jacquemond V, Casas M. J Gen Physiol (2021) 153(12):e202012635
- Detection of Ca2+ transients near ryanodine receptors by targeting fluorescent Ca2+ sensors to the triad. Sanchez C, Berthier C, Tourneur Y, Monteiro L, Allard B, Csernoch L, Jacquemond V. J Gen Physiol (2021) 153(4):e202012592
- In vivo RyR1 reduction in muscle triggers a core-like myopathy. Pelletier L, Petiot A, Brocard J, Giannesini B, Giovannini D, Sanchez C, Travard L, Chivet M, Beaufils M, Kutchukian C, Bendahan D, Metzger D, Franzini Armstrong C, Romero NB, Rendu J, Jacquemond V, Fauré J, Marty I. Acta Neuropathol Commun (2020) 8(1):192
- Differential physiological roles for BIN1 isoforms in skeletal muscle development, function and regeneration. Prokic I, Cowling BS, Kutchukian C, Kretz C, Tasfaout H, Gache V, Hergueux J, Wendling O, Ferry A, Toussaint A, Gavriilidis C, Nattarayan V, Koch C, Lainé J, Combe R, Tiret L, Jacquemond V, Pilot-Storck F, Laporte J. Dis Model Mech (2020) 13(11): dmm044354
- Divalent cations permeation in a Ca2+ non-conducting skeletal muscle dihydropyridine receptor mouse model. Idoux R, Fuster C, Jacquemond V, Dayal A, Grabner M, Charnet P, Allard B. Cell Calcium (2020) 91 : 102256
- Preserved Ca2+ handling and excitation-contraction coupling in muscle fibres from diet-induced obese mice. Jaque-Fernandez F, Beaulant A, Berthier C, Monteiro L, Allard B, Casas M, Rieusset J, Jacquemond V. Diabetologia (2020) 63: 2471-2481
- Molecular determinants of homo- and heteromeric interactions of Junctophilin-1 at triads in adult skeletal muscle fibers. Rossi D, Scarcella AM, Liguori E, Lorenzini S, Pierantozzi E, Kutchukian C, Jacquemond V, Messa M, De Camilli P, Sorrentino V. Proc Natl Acad Sci U S A (2019) 116(31): 15716–15724
- Unraveling the pathophysiology of Bethlem Myopathy using a unique zebrafish model for the disease.
Idoux R, Bretaud S, Berthier C, Jacquemond V, Ruggiero F, Allard B. Med Sci (2019) 35 (Hors série n° 2) : 39-42 - Mammalian skeletal muscle does not express functional voltage-gated H+ channels.
Fuster C, Idoux R, Berthier C, Jacquemond V, Allard B. Am J Physiol Cell Physiol. (2018) 315:C776-C779. - Tracking the sarcoplasmic reticulum membrane voltage in muscle with a FRET biosensor.
Sanchez C, Berthier C, Allard B, Perrot J, Bouvard C, Tsutsui H, Okamura Y, Jacquemond V. J Gen Physiol. (2018) 150:1163-1177. - Na leak with gating pore properties in hypokalemic periodic paralysis V876E mutant muscle Ca channel.
Fuster C, Perrot J, Berthier C, Jacquemond V, Charnet P, Allard B. J Gen Physiol (2017) 149:1139-1148. - Impaired excitation-contraction coupling in muscle fibres from the dynamin2R465W mouse model of centronuclear myopathy.
Kutchukian C, Szentesi P, Allard B, Trochet D, Beuvin M, Berthier C, Tourneur Y, Guicheney P, Csernoch L, Bitoun M, Jacquemond V.J Physiol (2017) 595:7369-7382.. - Elevated resting H+ current in the R1239H type 1 hypokalaemic periodic paralysis mutated Ca2+ channel.
Fuster C, Perrot J, Berthier C, Jacquemond V, Allard B. J Physiol (2017) ;595:6417-6428. - Phosphatidylinositol 3-kinase inhibition restores Ca2+ release defects and prolongs survival in myotubularin-deficient mice.
Kutchukian C, Lo Scrudato M, Tourneur Y, Poulard K, Vignaud A, Berthier C, Allard B, Lawlor MW, Buj-Bello A, Jacquemond V. PNAS(2016) 113:14432-14437. - Voltage-gated Ca2+ influx through L-type channels contributes to sarcoplasmic reticulum Ca2+ loading in skeletal muscle.
Robin G, Allard B. J Physiol (2015) 593:4781-4797. - Depression of voltage-activated Ca2+ release in skeletal muscle by activation of a voltage-sensing phosphatase.
Berthier C, Kutchukian C, Bouvard C, Okamura Y, Jacquemond V. J Gen Physiol (2015) 145:315-330. - Dihydropyridine receptors actively control gating of ryanodine receptors in resting mouse skeletal muscle fibres.
Robin G, Allard B. J Physiol (2012) 590:60275-6036. - Defects in Ca2+ release associated with local expression of pathological ryanodine receptors in mouse muscle fibres.
Lefebvre R, Legrand C, Gonz·lez-RodrÌguez E, Groom L, Dirksen RT, Jacquemond V. J Physiol (2011) 589:5361-5382.
FUNDING
- AFM-Téléthon / MyoNeurALP Alliance (2022-2027)
- ANR FishandCol6 (2020-2023)
- French Foundation for Medical Research
- Cancer League
- CONYCIT (Conicyt Comisión Nacional de Investigación Científica y Tecnológica)
![]()



Address
Institut NeuroMyoGène
UCBL – CNRS UMR 5310 – INSERM U1217
Faculté de Médecine et de Pharmacie – 3ème étage – Couloir AB
8 avenue Rockefeller
69008 Lyon
France

